The genus Paranthropus represents one of the most striking evolutionary experiments in hominin history. Between roughly 2.7 and 1.2 million years ago, three species of heavily built hominins occupied habitats across eastern and southern Africa, developing cranial and dental anatomy so extreme that early researchers dubbed one specimen "Nutcracker Man."1 Their enormous flat molars, thick enamel, massive jaw muscles, and prominent sagittal crests—bony ridges running along the top of the skull for muscle attachment—set them dramatically apart from the gracile australopiths that preceded them and from the genus Homo that evolved alongside them.2, 3 The Paranthropus lineage ultimately went extinct without descendants, but its fossil record provides crucial evidence that human evolution involved multiple coexisting lineages pursuing very different adaptive strategies.4
Discovery and naming
The first Paranthropus species to be recognized was P. robustus, discovered by the Scottish palaeontologist Robert Broom at Kromdraai, South Africa, in 1938. Broom received a partial skull and jaw (TM 1517) from a local schoolboy named Gert Terblanche, who had found it in a limestone quarry. Recognizing that the specimen's massive jaw and inflated cheekbones differed fundamentally from the more gracile Australopithecus africanus that Raymond Dart had described in 1925, Broom erected the new genus Paranthropus, meaning "beside human."5 Broom continued excavations in South Africa for over a decade, recovering additional P. robustus material from Swartkrans, including the well-preserved cranium SK 48, which he described in collaboration with John T. Robinson in 1952.6
Two decades later, the discovery of an East African counterpart transformed paleoanthropology. On 17 July 1959, Mary Leakey found a nearly complete cranium (OH 5) eroding from the surface at Olduvai Gorge, Tanzania. The skull's enormous sagittal crest and extraordinarily large molars immediately distinguished it from anything previously known. Louis Leakey initially placed the specimen in a new genus, Zinjanthropus boisei—"Zinj" for an old Arabic name for East Africa and "boisei" honouring the expedition's funder Charles Boise.1 The discovery attracted worldwide media attention and, crucially, secured funding from the National Geographic Society that would underwrite decades of Leakey family research in East Africa.7 Most researchers now classify OH 5 as Paranthropus boisei, recognizing its close relationship to Broom's South African genus.2
The third species, P. aethiopicus, was formally recognized after Alan Walker and Richard Leakey announced the discovery of KNM-WT 17000—the "Black Skull"—from west of Lake Turkana, Kenya, in 1985. Dated to approximately 2.5 million years ago, the specimen combined an extremely prognathic (forward-jutting) face and a tiny braincase of just 410 cubic centimetres with a massive sagittal crest, presenting a mosaic of primitive and derived features that suggested it was ancestral to P. boisei.8 Earlier fragmentary jaw material from the Omo Valley in Ethiopia, originally described by Camille Arambourg and Yves Coppens in 1968, was retrospectively assigned to this species.9

Anatomy and the chewing apparatus
The defining feature of all Paranthropus species is a suite of cranial and dental adaptations collectively termed the "masticatory complex"—an interlocking set of modifications that produced extraordinarily powerful chewing. The most visible of these is the sagittal crest, a ridge of bone running along the midline of the skull to which the temporalis muscles attached. In male P. boisei, this crest could extend several centimetres above the braincase, giving the skull a distinctive peaked profile.2 Broad, flaring zygomatic arches (cheekbones) provided additional space for enlarged masseter muscles, the other primary muscles of mastication.3
The teeth themselves are equally remarkable. Paranthropus boisei possessed the largest molars of any known hominin, with molar areas roughly four times those of modern humans. The premolars were "molarized"—broadened and flattened to resemble molars—while the anterior teeth (incisors and canines) were comparatively tiny, a pattern described as megadonty.10 The enamel coating these enormous teeth was exceptionally thick, in some cases exceeding 2.5 millimetres, which would have resisted heavy wear during prolonged chewing of tough or hard foods.11 The mandible (lower jaw) was deep and buttressed, with thick cortical bone capable of withstanding the tremendous forces generated during mastication.3
Despite these powerful jaws, Paranthropus brains remained small. Cranial capacities ranged from roughly 410 cc in P. aethiopicus to approximately 530 cc in P. boisei, comparable to chimpanzees and only modestly larger than earlier australopiths.8, 2 There is some evidence of a slight increase in brain size within the P. boisei lineage over time—roughly 100 cc over one million years—but this trend was far less dramatic than the concurrent brain expansion occurring in early Homo.10 The postcranial skeleton, where preserved, suggests a body mass of roughly 40–50 kg for males, with females considerably smaller, indicating marked sexual dimorphism.3
Cranial capacity across Paranthropus species8, 2, 12
Diet: not what the teeth suggest
For decades after the discovery of OH 5, the massive dental apparatus of Paranthropus was interpreted as an adaptation for crushing hard objects such as nuts, seeds, and bones—hence the popular nickname "Nutcracker Man." This intuitive interpretation began to unravel in the 2000s when new analytical techniques revealed a more complex picture. Dental microwear analysis, which examines microscopic scratches and pits on tooth surfaces left by the last meals consumed before death, showed that P. boisei teeth exhibit surprisingly low complexity and light pitting, resembling the wear patterns of modern leaf-eating primates rather than hard-object feeders.13
Stable carbon isotope analysis provided an even more startling revelation. By measuring the ratios of carbon-13 to carbon-12 in fossil tooth enamel, researchers can distinguish between diets based on C3 plants (most trees, shrubs, and forest fruits) and C4 plants (tropical grasses and sedges). A landmark 2011 study by Thure Cerling and colleagues found that P. boisei had a diet dominated by C4 resources to a degree unmatched by any other hominin, with C4 plants comprising roughly 77% of the diet.14 The most likely candidates are papyrus sedges and grasses, which would have been abundant in the wetland margins of East African lakes and rivers.14
This evidence reframed the massive chewing apparatus not as a nutcracker but as a grass-processing machine. The enormous molars, thick enamel, and powerful jaw muscles may have been adaptations for grinding large quantities of tough, low-quality vegetation for hours each day—an ecological strategy more analogous to a gorilla or a gelada baboon than to a squirrel cracking nuts.13, 14 The dietary picture for P. robustus in South Africa was somewhat different: isotope data indicate a mixed diet of predominantly C3 foods (fruits and leaves) with approximately 25–35% C4 resources, and microwear analysis shows more pitting, suggesting occasional hard-object feeding.15 A 2020 study by Luján and colleagues confirmed that the dietary shift toward C4 foods in eastern African Paranthropus occurred around 2.37 million years ago, coinciding with broader environmental changes across the continent.16
Key specimens
The fossil record of Paranthropus, while not as extensive as that of Australopithecus afarensis, includes several exceptionally important specimens that have shaped our understanding of robust hominin biology and evolution. The following table summarizes the six most significant finds.
Key Paranthropus specimens1, 5, 8, 17, 18, 19
| Specimen | Species | Age (Ma) | Site | Cranial capacity | Significance |
|---|---|---|---|---|---|
| KNM-WT 17000 | P. aethiopicus | ~2.5 | West Turkana, Kenya | 410 cc | Mosaic of primitive and derived features; probable ancestor of P. boisei |
| OH 5 ("Zinj") | P. boisei | 1.78 | Olduvai Gorge, Tanzania | ~530 cc | Holotype; massive sagittal crest and megadont molars |
| KNM-ER 406 | P. boisei | 1.7 | Koobi Fora, Kenya | 510 cc | Found near H. erectus cranium KNM-ER 3733; proved coexistence |
| TM 1517 | P. robustus | ~1.8 | Kromdraai, South Africa | — | Holotype of genus Paranthropus; Broom 1938 |
| SK 48 | P. robustus | ~1.65 | Swartkrans, South Africa | ~530 cc | Well-preserved cranium; key to understanding P. robustus morphology |
| DNH 7 | P. robustus | ~2.0 | Drimolen, South Africa | — | Most complete P. robustus skull; probable female |

The discovery of KNM-ER 406 by Richard Leakey's team at Koobi Fora in 1969 was a watershed moment. Found in close proximity to the Homo erectus cranium KNM-ER 3733, it provided direct evidence that Paranthropus boisei and early Homo occupied the same landscape at the same time.17 This finding demolished any remaining notion of a simple linear progression from "ape-man" to modern human, demonstrating instead that the hominin family tree was a bush with multiple contemporaneous branches.4
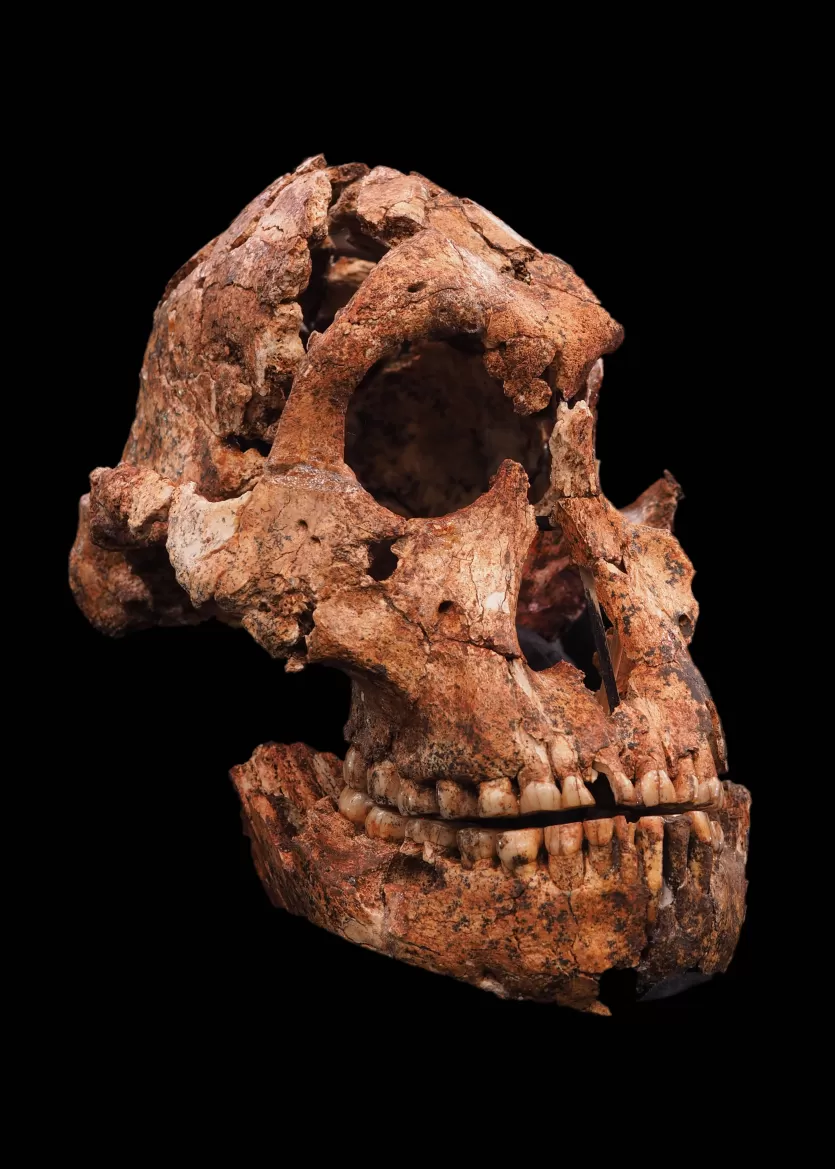
At Drimolen in South Africa, Andre Keyser's discovery of DNH 7 in 1994 provided the most complete P. robustus cranium ever found. The specimen's relatively gracile features—lacking the pronounced sagittal crest of male specimens—suggested it was female, offering important evidence for the degree of sexual dimorphism in the species.19 More recently, the discovery of DNH 155 at Drimolen, dated to approximately 2.04–1.95 million years ago, pushed the earliest occurrence of P. robustus back in time and documented microevolutionary change within the species.20
The Leakey family and Paranthropus
The story of Paranthropus is inseparable from the Leakey family, whose three generations of fieldwork in East Africa have shaped modern paleoanthropology. Louis and Mary Leakey had been excavating at Olduvai Gorge since the 1930s with limited funding and few spectacular finds. Mary Leakey's discovery of OH 5 on 17 July 1959 changed everything. Louis Leakey immediately recognized its significance and publicized the find with characteristic flair, securing a major grant from the National Geographic Society that would fund not only continued work at Olduvai but eventually support their son Richard's expeditions to Lake Turkana.7
Louis Leakey initially believed OH 5 was the toolmaker responsible for the Oldowan stone tools found at the same site. This interpretation was challenged when, in 1960, the Leakeys discovered fossils of a more gracile hominin at Olduvai—eventually named Homo habilis—that Louis argued was the true toolmaker, relegating Paranthropus to the role of a specialized evolutionary dead end.7, 21 The debate over whether Paranthropus made or used stone tools continues, though most researchers now attribute Oldowan tool production primarily to early Homo.4
Richard Leakey continued his parents' legacy at Koobi Fora on the eastern shore of Lake Turkana, where his team recovered numerous P. boisei specimens through the 1970s and 1980s, including KNM-ER 406 and KNM-ER 732. These discoveries, combined with Alan Walker's recovery of the Black Skull (KNM-WT 17000) on the western shore in 1985, established the Lake Turkana Basin as the richest source of Paranthropus fossils in the world.17, 8 Meave Leakey, Richard's wife, and their daughter Louise Leakey have continued fieldwork in the region into the twenty-first century, recovering additional hominin material that has refined the chronology and diversity of the Turkana fossil assemblage.22
Coexistence with early Homo
One of the most important lessons from the Paranthropus fossil record is that for more than a million years, multiple hominin species shared the African landscape. Between approximately 2.0 and 1.5 million years ago, at least three hominin lineages—P. boisei, Homo habilis (or H. rudolfensis), and Homo erectus—occupied overlapping ranges in East Africa.4, 17 In South Africa, P. robustus coexisted with early Homo at sites such as Swartkrans and Drimolen.6, 20
This coexistence was possible because the lineages appear to have exploited different ecological niches. Isotope and microwear evidence indicates that P. boisei specialized in C4 grasses and sedges in wetland-adjacent habitats, while early Homo consumed a broader omnivorous diet that increasingly included animal protein.14, 23 The analogy is sometimes drawn to modern African ecosystems where multiple primate species coexist by partitioning food resources: baboons, chimpanzees, and gorillas can all occupy the same forest because they emphasize different parts of the available food base.4
At Swartkrans, excavations led by C. K. Brain recovered bone tools associated with P. robustus deposits, raising the possibility that robust australopiths used simple bone implements for digging termite mounds or extracting tubers. Brain's team recovered 84 bone tools from Swartkrans and Keyser later found 23 more at Drimolen.24 Whether Paranthropus made these tools or whether they were produced by the early Homo individuals also present at these sites remains debated. Evidence of controlled fire use at Swartkrans, dated to roughly 1.0 million years ago, is generally attributed to Homo rather than Paranthropus.24
Taxonomy and evolutionary relationships
Whether the three Paranthropus species form a true evolutionary group (a monophyletic clade) or represent independent acquisitions of "robust" features from different australopith ancestors remains one of the enduring debates in paleoanthropology. The question matters because it determines whether "Paranthropus" is a valid genus or merely a convenient label for convergently evolved species that should be placed back in Australopithecus.25
Most cladistic analyses have supported monophyly, finding that the shared derived features of the masticatory complex—sagittal cresting, molarized premolars, megadont molars, thick enamel, and robust mandibles—are most parsimoniously explained by common descent rather than convergent evolution.10, 25 Wood and Constantino, in their 2007 review of fifty years of P. boisei research, concluded that the evidence favours recognizing Paranthropus as "an adaptively distinctive monophyletic group," while acknowledging that the phylogenetic position of P. aethiopicus as a basal member of the clade still requires further testing.10
The prevailing phylogenetic hypothesis places P. aethiopicus as the earliest and most primitive member of the clade, giving rise to P. boisei in East Africa by approximately 2.3 million years ago. The relationship of P. robustus in South Africa is less clear: it may have diverged from the same ancestor as P. boisei, or it may represent an independent offshoot of a late Australopithecus population.8, 25 The ancestral species from which Paranthropus evolved is also debated, with Australopithecus afarensis and A. africanus both proposed as candidates.4
Extinction and its causes
The last known Paranthropus fossils date to approximately 1.2 million years ago, after which the genus disappears from the fossil record entirely. The extinction of Paranthropus coincided with a period of intensifying climatic variability across Africa, and most researchers link the two. Peter deMenocal's analysis of deep-sea sediment cores off the African coast revealed that dust flux—a proxy for aridity—increased markedly around 1.7 million years ago and again near 1.0 million years ago, reflecting a shift toward more extreme oscillations between wet and dry conditions.26
The "variability selection" hypothesis, proposed by Rick Potts of the Smithsonian Institution, suggests that the key selective pressure during this period was not any single environmental state but the increasing amplitude of environmental fluctuations themselves. Species with narrow ecological tolerances—dietary specialists like P. boisei, which depended heavily on C4 wetland vegetation—would have been particularly vulnerable when their preferred habitats contracted during arid phases.27 By contrast, the genus Homo, with its broader diet, larger brain, increasing reliance on technology, and evidence of greater geographic mobility, was better equipped to cope with unpredictable environments.23
The extinction of Paranthropus was part of a broader faunal turnover in Africa around this time. Several other large mammal lineages also disappeared, suggesting that the environmental changes were severe enough to restructure entire ecosystems rather than targeting a single hominin lineage.26 Competition with Homo may have played a secondary role, particularly if expanding Homo erectus populations encroached on Paranthropus habitats or depleted shared resources, but direct evidence for competitive exclusion remains limited.4
Significance for human evolution
The Paranthropus lineage matters for understanding human evolution in several ways. First, it demonstrates that the hominin family tree was not a ladder but a bush. For most of the Pleistocene, being a hominin did not mean being on the path to Homo sapiens; multiple lineages coexisted, each pursuing distinct adaptive strategies, and most of them went extinct.4 The fact that our own genus survived while Paranthropus did not was not inevitable but contingent on ecological circumstances and the particular traits—dietary flexibility, technological capacity, social complexity—that Homo happened to evolve.27
Second, Paranthropus provides a natural experiment in the evolutionary consequences of dietary specialization. The genus invested heavily in a single adaptive solution—powerful chewing of tough, low-quality plant foods—and this strategy sustained three species across two continents for over 1.5 million years, a duration that dwarfs the 300,000-year history of Homo sapiens.2, 14 That Paranthropus ultimately went extinct does not mean the strategy was a failure; it means that even successful adaptations can become liabilities when environments change faster than lineages can respond.27
Third, the coexistence of Paranthropus and early Homo at the same fossil sites provides direct evidence against the creationist claim that hominin fossils represent either "fully human" or "fully ape" specimens with no intermediates. The Paranthropus species are unambiguously neither: they walked bipedally like humans, had brains comparable to great apes, possessed dental anatomy unlike any living primate, and occupied an adaptive zone that no longer exists.3, 4 They are, in the most literal sense, creatures that make no sense outside an evolutionary framework.